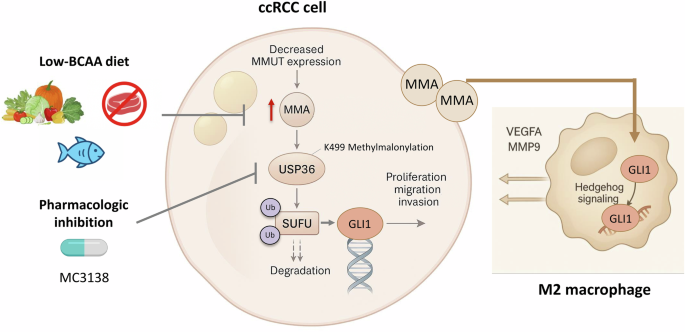
If MMA fuels malignancy, could its depletion starve it? Miao et al. [5] take their fundamental concepts and apply them in a clever intervention strategy. They took inspiration from the clinical dietary management of patients with methylmalonic acidemia and placed ccRCC-bearing mice under a low-BCAA diet to reduce MMA availability. This simple dietary change worked therapeutically to not only diminish tumor growth but also reduce pulmonary metastases and limit M2 macrophage infiltration. This reinforces the idea that a net metabolic shift in the diet can therapeutically restrain the immune system, and whether MMA could contribute to immune evasion or resistance to immunotherapies should be investigated.
Therapeutic nutrient restriction has often been considered impractical, yet recent studies on serine/glycine and methionine metabolism show that selective metabolic vulnerabilities can provide opportunities for therapeutic exploitation [12, 13]. The low-BCAA diet discussed in this context will also be a feasible clinical adjunct to treatment for many, and BCAA restriction will be less demanding for patients, as it can be transient and modulated as needed.
Although elegant, there are a number of unanswered questions. First, what is the mechanistic reason for the observed levels of MMUT being decreased in ccRCC. Second, proteomic screens have identified numerous methylmalonyl-lysine sites [14]; thus, metabolic off-targeting may have wide-ranging effects on metabolism. On the translational side, there are several important questions: (1) Is a measurement of MMA levels useful as a biomarker of aggressive disease, or as a measure of changes in the tumor microenvironment (TME) or metabolic vulnerability to therapy? (2) Where is MMA spatially distributed within the tumor and stroma? (3) How practical is a low-BCAA diet as a therapeutic approach?
On the clinical side, the use of BCAA restriction as a form of therapy in human patients with ccRCC is a long way from being practiced due to the metabolic and skeletal muscle comorbidities that are present in RCC patients. The drug MC3138 is effective in mice, but MC3138 has not been evaluated regarding its pharmacokinetic profile or its toxicity in humans. As such, the concept of “metabolic detuning”, or lowering the production of a metabolite that is toxic to the cell, is certainly an interesting approach that may be used to develop new combinations of therapies, including those using immune checkpoint inhibitors or anti-angiogenic drugs.
In summary, the current study clearly demonstrates that ccRCC is a “metabolic” disease and not simply a result of signaling pathway alterations. In addition, this work further demonstrates that the metabolic landscape of tumors is not random and may reflect the cellular wiring of the tumor cells, which may provide additional points of intervention beyond the information provided by genomic studies.